The Hendrickson Group







GPI Membrane Anchoring of Proteins
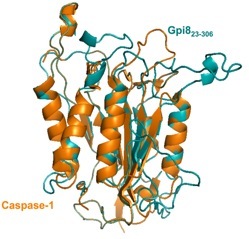
Glycosylphosphatidylinositol (GPI) membrane anchors are complex glycolipids that are post-translationally attached to the C-termini of as many as 1% of eukaryotic proteins. Upon anchor attachment, these modified proteins are translocated to the outer cell wall where they play a variety of important cell surface functions. Our group investigates GPI transamidase (GPI-T), the enzyme that attaches GPI anchors to proteins. GPI-T is a complex enzyme, comprised of at least five subunits, all of which are membrane-bound.
We are interested in the characterization of GPI-T, both in vivo and in vitro. We are developing new tools to study this enzyme and new methodologies for its solubilization. We also seek to minimize GPI-T into a soluble enzyme that can be used to modifiy protein substrates with non-natural compounds of biophysical importance. Click HERE for more information on this project.
Indirect tRNA Aminoacylation Pathways
The aminoacyl-tRNA synthetases catalyze the highly accurate attachment of specific amino acids to specific tRNAs. The precision of these enzymes is foundational to accuracy in translation of the genetic code. Many organisms lack a complete set of aminoacyl-tRNA synthetase but still incorporate all 20 amino acids into proteins. In these species, one or more aminoacylated-tRNAs are biosynthesized indirectly.
We are investigating the indirect biosynthesis of Gln-tRNAGln and Asn-tRNAAsn in the pathogenic bacterium Helicobacter pylori. H. pylori is missing both glutaminyl-tRNA synthetase and asparaginyl-tRNA synthetase. We are interested in understanding the evolution of direct versus indirect tRNA aminoacylation pathways as well as the mechanisms that are used by H. pylori to prevent misacylated tRNAs from entering the ribosome prior to conversion to their accurately aminoacylated counterpoints. Click HERE for more information on this project.






















