The Hendrickson Group







Indirect tRNA Aminoacylation
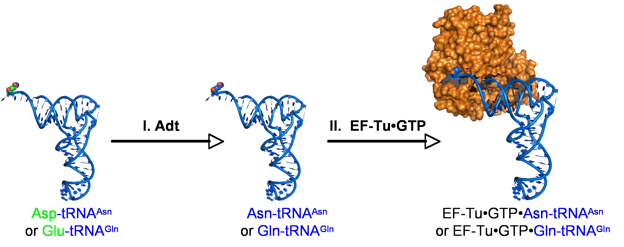
Many bacteria and archaea lack a full set of aminoacyl-tRNA synthetases. In fact, glutaminyl-tRNA synthetase and asparaginyl-tRNA synthetase could be consider rare. When these enzymes are missing, their products, Gln-tRNAGln and Asn-tRNAAsn are biosynthesized via a two-step, indirect pathway that requires the action of a misacylating tRNA synthetase and an amidotransferase (Adt).
Our research group is characterizing this system of misacylation and repair in the pathogenic bacterium Helicobacter pylori, a bacterium that is missing both AsnRS and GlnRS.
Evolution of GluRS2: H. pylori uses an unusual misacylating glutamyl-tRNA synthetase, GluRS2. This enzyme is uniquely specific for misacylation of tRNAGln and has lost the ability to aminoacylate its “cognate” tRNAGlu. We have proposed that GluRS2 represents an effort by bacteria like Hp to evolve a novel bacterial GlnRS. Using molecular modeling, site-directed mutagenesis and targeted evolution, we are probing this evolutionary pathway, both in terms of what might have happened and what still might happen.
Adt and Transamidation of Misacylated tRNAs: Adt is responsible for repairing both Asp-tRNAAsn and Glu-tRNAGln, but never mistakenly transamidates Asp-tRNAAsp or Glu-tRNAGlu. This enzyme is a dimer of a heterotrimer and is known to form a complex with archaeal type ND-AspRSs. We are interested in the enzymology of this enzyme and the formation of Adt-containing complexes.
Translational fidelity: Translation of the genetic code occurs with high fidelity in Hp, despite the presence of ND-AspRS and GluRS2. Our lab has a long-standing interest in fidelity mechanisms. With this in mind, we are studying EF-Tu, in conjunction with Adt, to understand how Hp prevents translational errors from occurring at Asn and Gln codons.


























