The Hendrickson Group







GPI Membrane Anchoring of Proteins
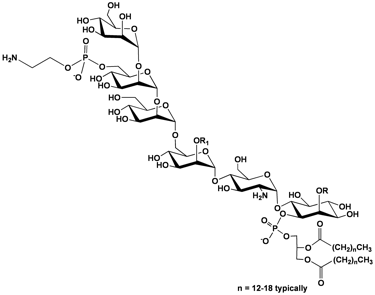
Glycosylphosphatidylinositol (GPI) membrane anchors are complex glycolipids that are post-translationally attached to the C-termini of as many as 1% of eukaryotic proteins. Upon anchor attachment, these modified proteins are translocated to the outer cell wall where they play a variety of important cell surface functions. Our group investigates GPI transamidase (GPI-T), the enzyme that attaches GPI anchors to proteins. GPI-T is a complex enzyme, comprised of at least five subunits, all of which are membrane-bound.
An in vitro assay for GPI-T: In vitro efforts to characterize GPI-T have been hindered by the complexity and solubility of this enzyme and by the lack of a quantitative high-throughput assay. We are developing just such an assay for GPI-T using synthetic peptides and minimalist GPI anchor mimics (both commercial and synthetic). This assay will be applied to a wide variety of experiments to characterize GPI-T, including the identification of substrate recognition motifs and to assess in vitro reconstitution of GPI-T.
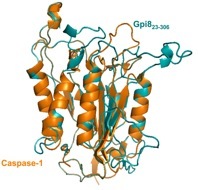
Solubilization and analysis of individual subunits: GPI-T is composed of five different subunits. We have already demonstrated that Gpi8, the active site subunit, exists as a homodimer, much like caspases (proteases that exhibit sequence and apparent structural similarity to Gpi8). We are now expanding these efforts to include analyses of the different subunit soluble domains as well as the full-length, membrane-associated subunits.
In vitro reconstitution of GPI-T, one subunit at a time: The Holy Grail of GPI-T research will be the demonstration of activity with pure, reconstituted or co-purified enzyme, using our in vitro assay. Such an experiment would prove that the five known subunits (or a subset thereof) are necessary and sufficient for enzyme activity. Furthermore, pure, active enzyme would open up innumerable different types of experiments to truly understand this complicated system.
Synthesis and testing of minimalistic GPI anchor mimics: As a counterpoint to our assay development, we are synthesizing a series of truncated GPI anchors as substrates for GPI-T.




